- الحج والعمرة
-
عن الهيئة
الهيئة في رؤية 2030
إستراتيجية الهيئة
التوظيف والحياة في الهيئة
- القوائم المعلوماتية
-
المجالات
- ركن المستهلكين
- المركز الإعلامي
- الخدمات الإلكترونية
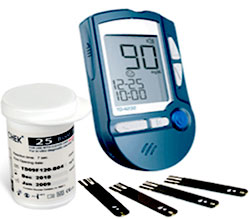
Saudi Food and Drug Authority is publishing an announcement about Blood Glucose Meters Model CLEVER CHEK TD-4232
Saudi Food and Drug Authority is publishing an announcement about Blood Glucose Meters Model CLEVER CHEK TD-4232
Saudi Food and Drug Authority is publishing an announcement about Blood Glucose Meters Model CLEVER CHEK TD-4232
2012-06-25
Saudi Food and Drug Authority would like to warn healthcare practitioners, and all patients about Blood Glucose Meters Model CLEVER CHEK TD-4232 Manufactured by TaiDoc Technology – in Taiwan , may not alarm if insufficient blood is applied .
This meter doesn’t have a blood glucose test strip under-fill detection mechanism, nor does it provide feedback to the users when sufficient blood has been applied. This meter does not alert users when insufficient blood has been applied.
Potential for falsely low blood glucose results if the test strips are under-filled, and falsely high results if over-filled. This could result in inappropriate insulin administration.
SFDA assure that CLEVER CHEK blood glucose meter hasn’t obtain market authorization from SFDA and hasn’t been cleared via KSA ports of entry.
Saudi Food and Drug Authority recommend to use alternative blood glucose monitoring devices, possibly with feedback and/or large display fonts.
Saudi Food and Drug Authority encourages all healthcare practitioners and all patients to Report any adverse event associated with medical devices through the National Center for Medical Devices Reporting : http://ncmdr.sfda.gov.sa