An Evaluation for the Safety of Heater-Cooler Device
An Evaluation for the Safety of Heater-Cooler Device
Heater-Cooler device is a unit that is coupled with Heart-Lung Machine through closed circuit, which is used to cool or warm patients during heart-lung surgeries. This device is believed to cause a risk of developing Nontuberculous mycobacteria (NTM) infection in patients undergoing cardiothoracic surgeries, as revealed by recently published reports around the world [1].
The study was conducted to attain the following objectives:
- Evaluate the risks associated with heater cooler devices, as indicated by the published safety communication.
- Derive recommendations to increase the level of safety and quality of the current practices of the HCD within Saudi healthcare facilities.
Heater-Cooler device (HCD) is used to cool or warm patients during surgeries involving heart and lung as cardiothoracic surgeries. HCD is coupled with Heart-Lung Machine through closed circuit. The circuit is intended to supply temperature-controlled water from water tank to heat exchangers, which meant to retain the temperature of organ and blood at certain level as shown in figure 1 [1].
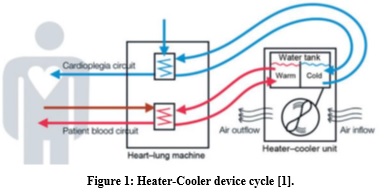
This mechanical technique is intended to preserve the stability of internal body temperature while external changes to body temperature are observed. HCD is classified as class II device, which refers to a moderate to high-risk medical device, and could be used for a variety of clinical procedures [1].
Since 2015, the U.S. Food and Drug Administration (FDA) has suspected a potential risk of Nontuberculous mycobacteria (NTM) infections after undergoing cardiothoracic surgeries comprising the use of HCD. Water tank within the HCD is believed to be the root cause of growing NTM bacteria through several aspects, including HCD design, aerosolization, and laminar flow disruption [2]. Efficient HCD includes water and air filters, which aimed to remove the NTM bacteria. Water filter has the ability to detect and remove the NTM existed into the water particles, while in some HCD air filter is not able to capture the diffused NTM in the air. Which then, Heater-Cooler device’s fan may ease the path of the inside non-filtered NTM to the sterilized filed.
Water tank is not airtight, which facilitates spreading the aerosolized NTM that already grown in water droplets, through the Heater-Cooler’s fan, and then traveled out the vent into the air. In fact, these droplets may enter the patient cavity if they were suspended in the air [2]. During surgery, laminar airflow serves as protection engineering controlled path to decrease airborne contaminants around patient; however, HCD exhaust fan may interrupt its nature depending on direction and distance of the exhaust, which then could carry NTM to the surgical filed [2].
It was reported by FDA that NTM is naturally diffused in the environment, so it is not a serious bacteria, but rarely can affect negatively as infections to some cases [3]. Consequently, a risk analysis report was developed to detail incidences and risks associated while using HCD. Not only that, but a safety communication also was published to inform Saudi healthcare facilities about the infectious issues while using HCD [4].
Part I: Methodology:
Three methods were merged to meet the scientific evaluation criteria along with the study objectives as the following subsequent: A screening over several SFDA databases, such as Medical Devices Marketing Authorization (MDMA) database, Port of entry, National Centre for Medical Devices Reporting (NCMDR), with a purpose of identifying the market data for heater cooler devices in Saudi Arabia that can enable reaching out the devices' users [4]. Then, each clinical user who use the device in question in the Saudi sites was requested to fulfill a survey that was designed to meet the study objectives.
Part II: Results:
- The Saudi market analysis revealed a total of 42 Saudi healthcare facilities that have HCD in use, and 94 % of these HCF are using one specific brand of HCD.
- The surveyed clinical users, have reported and upraised 5 cases of NTM infection.
- Responses show that 56% of the surveyed Saudi healthcare facilities are unaware in which part of HCD lead to serious infection.
- A percentage of 39% of the surveyed users have not adopted the manufacturer corrective actions to avoid the risk of NTM infection.
- Also, 6% of the surveyed healthcare providers were not aware of the SFDA published recommendations.
Part III: Conclusion
Recommendations for SFDA actions
- Manufacturer authorized representative SHALL provide a plan to increase the awareness of the device users regarding the risks associated with the device and the proper practices to avoid or reduce the risks as indicated by the updated IFU and the manufacturer recommendations.
- This plan is to be reviewed by the SFDA staff, and whenever approved, the manufacturer is committed to apply it in all sites that include the device in question.
- Manufacturer authorized representative SHALL provide the SFDA staff with a report of investigation for the 5 cases reported by the Saudi sites, which were shared previously with them through the email, though which the report SHALL include applying a corrective action plan for each individual case.
This study is authored by Eng. Jumana Masoudi, who designed the survey, collected and analyzed data, and wrote up the manuscript of the study. Eng. Bader Aloufi verified the study methodology and supervised the study progress. With the appreciation to the post-market clinical evaluation team for their efforts in conducting this work.
For further information or inquiries related to this study, you may contact us at: cia.md@sfda.gov.sa
[1] USFDA, "What is a Heater-Cooler Device?, " 11 06 2018. [Online]. Available: https://www.fda.gov/medical-devices/cardiovascular-devices/what-heater-cooler-device.
[2] USFDA, "FDA's Ongoing Evaluation and Continued Monitoring of Reports of Nontuberculous Mycobacteria Infections Associated with Heater-Cooler Devices," 30 09 2020. [Online]. Available: https://www.fda.gov/medical-devices/what-heater-cooler-device/fdas-ongoing-evaluation-and-continued-monitoring-reports-nontuberculous-mycobacteria-infections.
[3] USFDA, "Potential Risk of Infection during Cardiac Surgery When Using the CardioQuip Modular Cooler-Heater Device – Letter to Health Care Providers," 30 09 2020. [Online]. Available: https://www.fda.gov/medical-devices/letters-health-care-providers/potential-risk-infection-during-cardiac-surgery-when-using-cardioquip-modular-cooler-heater-device?utm_medium=email&utm_source=govdelivery.
[4] A. Alamri, "Risk Analysis Report: Heater-Cooler Devices," SFDA, Riyadh, 2019.


