Post-Market Clinical Evaluation Study for the Safety and Effectiveness of Inhaled Nitric Oxide Therapy
Post-Market Clinical Evaluation Study for the Safety and Effectiveness of Inhaled Nitric Oxide Therapy
Inhaled nitric oxide (iNO) therapy systems are a newly-developed technology for pulmonary vasodilation. This technology is used in several pulmonary conditions to improve the oxygenations as a rescue technique, beside the usage as a diagnostic approach in the pulmonary artery measurements. The aim of this study is to evaluate the safety of the iNO therapy in general, giving a closer look at the recent studies that investigate the roles of NO in the various medical conditions. The study also aims to enlighten the healthcare community about the current international practices in the iNO therapy at the one hand, and to define the known use problems and side effects associated with the technique on the other hand.
INO therapy
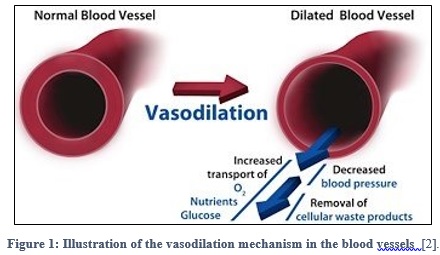
Nitric Oxide, or simply NO, is a colorless, odorless gas, which is known to be a potent and selective pulmonary vasodilator [1] –Vasodilators are the agents that aid in the dilation or widening of the blood vessels, which decreases blood pressure, due to the relaxation of smooth muscle cells within the vessel walls as shown in figure 1- [2]. NO is considered as an ideal pulmonary vasodilator for two reasons. First, it is a gas, and thus provides the ideal way to be delivered to the target blood vessels in the lung with avoiding systemic hypotension. Secondly, NO has high affinity for heme protein, and as a result of this, any excess inhaled NO in the lung would rapidly binds to hemoglobin, eliminating the potential for systemic vasodilatation. Therefore, this feature enables NO to be a pulmonary-specific vasodilator. [3]
Alternative approaches for INO therapy
Abrupt discontinuation of vasodilators is reported by the US FDA to cause rebound pulmonary hypertension, [4] which reflect the necessity behind the suggestions of using delivery systems to administer the gas, in order to supply the patients with constant dosage, and to ensure the continuous delivery. [5] Even though INO is the only pulmonary vasodilator approved by the US Food and Drug Administration for the treatment of persistent pulmonary hypertension (PPHN), the off-label use of other inhaled pulmonary vasodilators systems has been reported, [5] such as the inhaled aerosolized pulmonary vasodilators using prostacyclins, epoprostenol, [6] iloprost, [7] [8] and prostaglandin. While prostacyclins, epoprostenol, and iloprost have been approved to treat the pulmonary arterial hypertension (PAH), prostaglandin is approved for palliative therapy to maintain patency of the ductus-arteriosus in neonates. [5] However, these vasodilators are still not approved for the use with PPHN because they are supplied through nebulizers, and thus, lack the consistency in the drug delivery, which do not fulfill the requirement of preventing the rebound pulmonary hypertension. [5]
NO is described to associate with minimal toxicity, especially when administered in dosage of 20 ppm, and up to 40 ppm. [9] Yet, the main concerns involved with iNO toxicity include the excess production of NO2 and the elevated formation of methemoglobin. [3] [9] NO2 is a cytotoxic molecule, and can cause pulmonary injury at concentrations greater than 5 ppm. Its production is susceptible to occur when NO react with O2 in high oxygen concentration in the ventilator circuit. However, studies show that iNO at doses less than 80 ppm is not associated with significant NO2 productions. Methemoglobin can also be formed, due to the high affinity of NO to bind to the heme protein, and thus, methemoglobin levels should be measured frequently and kept at a level below 2.5%. Nevertheless, studies show that when iNO doses is maintained below 20 ppm, methemoglobin level is rarely raised above 2.5%. [3] [9]
INO therapy is approved by the US FDA for the treatment of term and near-term (> 34-weeks gestation) neonates with hypoxic respiratory failure associated persistent pulmonary hypertension PPHN. [1] Nonetheless, there are many other off-label uses of iNO, [10] including acute respiratory distress syndrome (ARDS) in infants and adults, [11] mitral stenosis and severe PH following cardiac surgery, [12] severe acute submissive pulmonary embolism, [13], beside other conditions. It is also used in several pulmonary conditions to improve the oxygenations as a rescue technique, beside the usage as a diagnostic approach in the pulmonary artery (PA) measurements.
Part 1: Clinical paper review
1.1 An overview of the search criteria
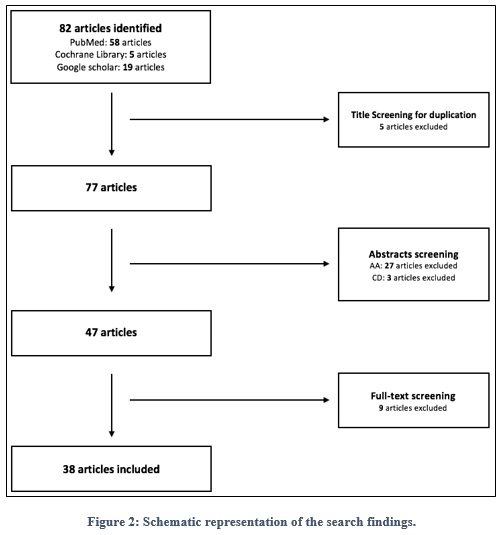
A literature review was conducted, with intention to acquire the most relevant papers that discuss the safety of iNO. A total of 82 articles were obtained and screened, which resulted in 38 articles, as shown in figure 2.
2.2 Results of the clinical paper review
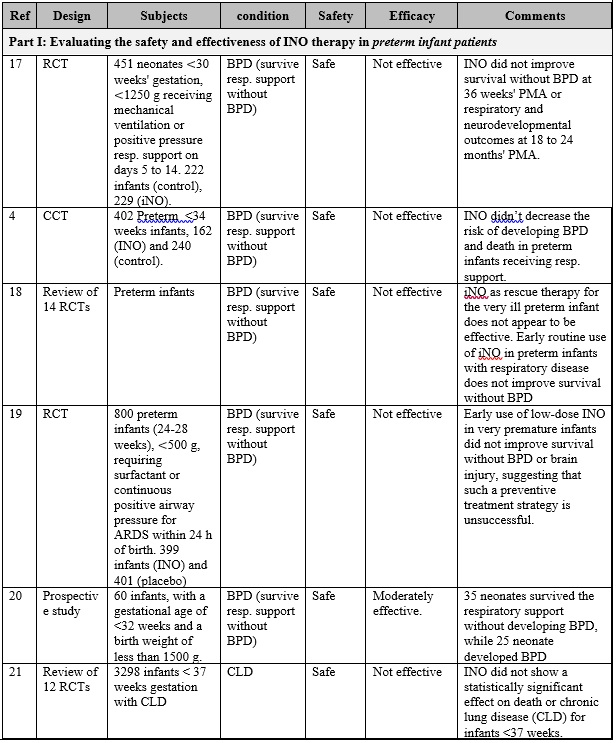
INO therapy was found to be administered to patients with different medical conditions and different populations (part I: preterm infants, part II: full-term infants, and part III: adults), as highlighted in table 1. Overall, it is clearly noticed that the use of iNO is mostly safe, and does not lead to severe side effects when used in tertiary care settings with strict administration protocols and careful monitoring. However, minimal toxicity might occur due to the formation of NO2 and methemoglobin when higher than recommended doses are administered, especially with preterm infants. [14] [15] Also, there is a strong evidence that iNO is associated with increasing the risk of the renal dysfunction with adult patients. [16] On the other hand, and with respect to the effectiveness of iNO therapy, it greatly varies based on the targeted patient populations.
The overall results of are presented in table 1, and can be summarized in the following points:
First: Current-evidence in the efficacy of iNO therapy with preterm infants:
- Generally speaking, iNO does not seem to be effective either as a rescue treatment option or as a routine treatment option for preterm infants who require ventilation assistant, where multiple studies (Ref. 17, 4, 18, 19, 20, and 21) have shown that iNO did not correspond to survive the respiration support without developing BPD, and showed no effect on reducing mortality.
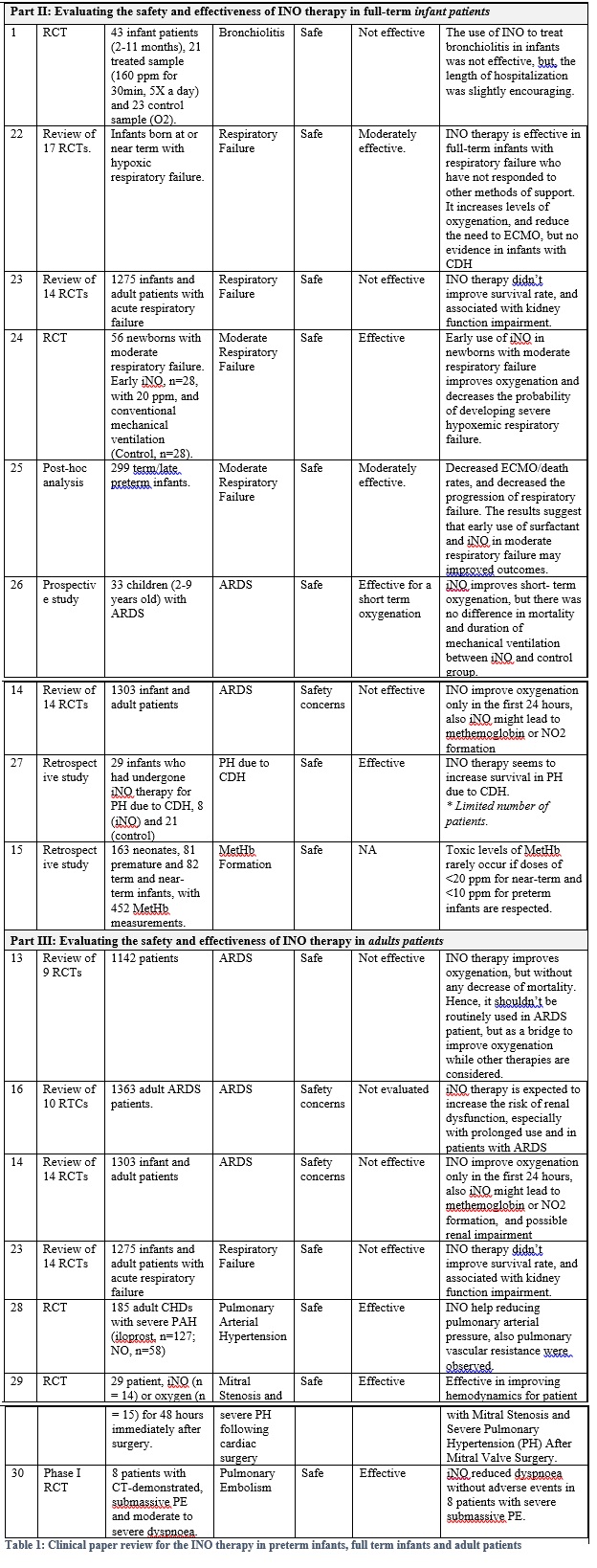
Secondly: Current-evidence in the efficacy of iNO therapy with full-term infant and kids:
- Overall, iNO therapy is effective as a rescue treatment in full-term infants with hypoxemic respiratory failure associated with PPHN who have not responded to other methods of support, where it increases levels of oxygenation, and reduce the need to ECMO, but no evidence in infants with CDH, (Ref. 22, 24, and 25). Also it aids in decreasing the probability of developing severe hypoxemic respiratory failure in full-term infants with moderate respiratory failure (Ref. 24, 25)
- Similarly, iNO therapy is effective as a rescue treatment in full-term infant and children with ARDS just for the short-term improvement in the oxygenation (Ref. 26 and 14).
Thirdly: Current-evidence in the efficacy of iNO therapy with adults:
- Overall, iNO therapy improves oxygenation, but without any decrease of mortality in adults with ARDS. Hence, it should be used as a rescue treatment, and as a bridge to improve oxygenation while other therapies are considered (Ref. 13 and 14)
Also, iNO shows a promising outcomes in reducing pulmonary arterial pressure in adult. (Ref. 28)
Part 2: Clinical experience review
This section aims to present the recommendations of some specialized international associations including: The National Institute of Health, The American Academy, The American Association for Respiratory Care, and The Canadian Pediatric Society. These parties published numerous guidelines to define the best practices in the utilization of the INO therapy, as summarized in Table 2.
Table 2: A complementary material addressing certain recommendations for the best-practices in using NO
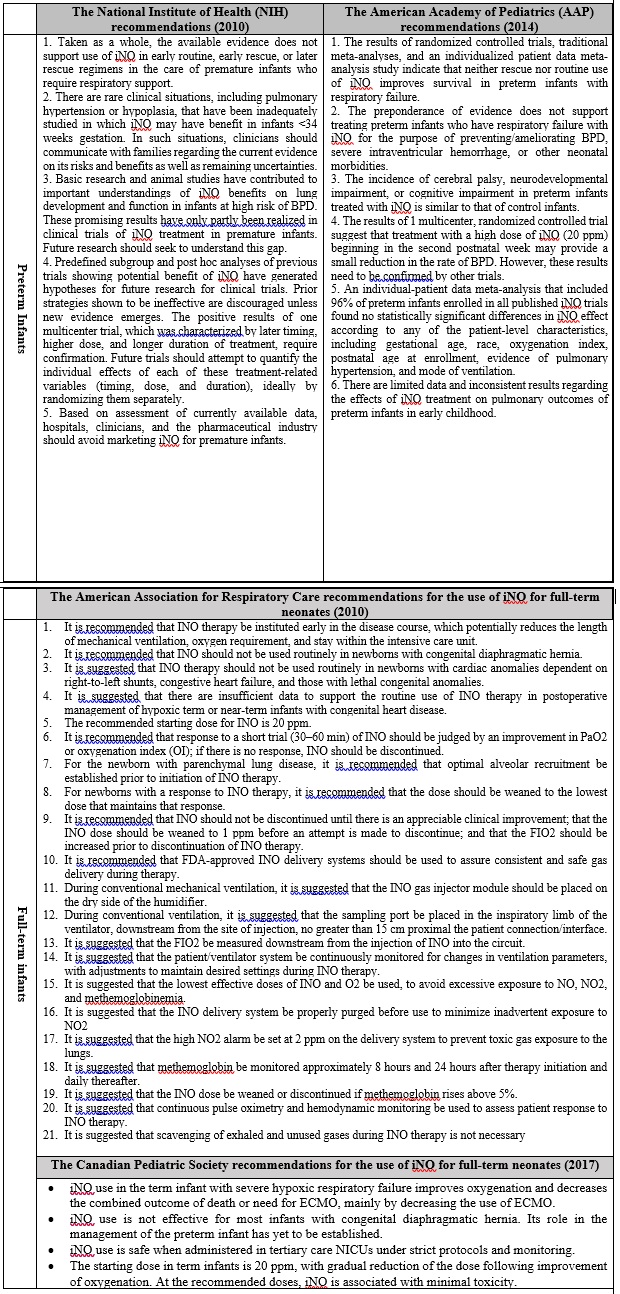
- It is suggested that inhaled nitric oxide (INO) be delivered through delivery systems to assure consistent and safe gas delivery during therapy, as the conventional way of delivering the gas through regulators and flowmeters are neither accurate, nor safe to the operators and the surrounding area.
- It is suggested that INO intended department adapts a clear policy and procedures for the administration of nitric oxide, to ensure that clinical staff understand and acquire the skills to administer the INO.
- INO should be used in a well-ventilated area, to avoid the possible build-up of NO2 in the area, as NO2 is cytotoxic and can cause pulmonary injury at concentrations >5 ppm. Also, while the INO systems are in use, a routine monitor of the NO2 level using NO2 detector should be considered, with an attention to maintain the level below 2.5 ppm.
-
INO is reported to associate with minimal toxicity when used as recommended. However, there is an evidence that the treatment could lead to the following potential adverse events whenever misused:
- Rebound Pulmonary Hypertension, which can be caused by the abrupt discontinuation of INO.
- Increased level of methemoglobin, which can be caused when doses >
20 ppm of INO is used.
- Increased levels of NO2, which can be caused when doses >
80 ppm of INO is used.
- Increase the risk of renal dysfunction, especially with prolonged INO use in adults with ARDS
Grateful thanks to Eng. Bader Aloufi for designing, reviewing the up to date articles, and writing up the context of this study. Thanks to Sara Alharthi for writing up this summary, with the appreciation to the post-market clinical evaluation team for their supports in conducting this work.
We also acknowledge the following members for sharing valuable inputs in evaluating the safety of iNO:
- Mr. Tariq Aljasser, the head of the respiratory care services at KFSH-RC.
- Mr. Essam Aljamhan, the head of the respiratory care services at KSUMC.
- Dr. Abdulrahman Alnemri, professor of pediatrics at KSU, consultant neonatologist, the head of NICU at KSUMC, and the former chairman of pediatric department at KSU.
- Dr. Eisa Sultan, consultant neonatologist, the head of NICU at Ohud hospital, and supervisor of neonatology improvement program of MOH at western region.
For inquiries related to this study, you may reach us through this email: cia.md@sfda.gov.sa
[1] Robert M DiBlasi RRT-NPS FAARC, Timothy R Myers RRT-NPS, and Dean R Hess PhD RRT FAARC, "Evidence-Based Clinical Practice Guideline: Inhaled Nitric Oxide for Neonates With Acute Hypoxic Respiratory Failure," RESPIRATORY CARE, vol. 55, no. 12, p. 1717, 2010.
[2] RRT, Mark S Siobal, "Pulmonary Vasodilators," RESPIRATORY CARE, vol. 52, no. 7, p. 885, 2007.
[3] G. G. K. a. K. P. V. M. Gregory M. Sokol, "Inhaled Nitric Oxide Therapy for Pulmonary Disorders of the Term and Preterm Infant," Semin Perinatol, vol. 40, no. 6, pp. 356-369, 2016.
[4] U.S. Department of Health and Human Services, Food and Drug Administration, "Guidance for Industry and for FDA Reviewers. Guidance Document for Premarket, Notification Submissions for Nitric Oxide, Delivery Apparatus, Nitric Oxide Analyzer, and Nitrogen Dioxide Analyzer," The US FDA, 2000.
[5] Nathan Cosa, Edward Costa Jr, "I nhaled pulmonary vasodilators for persistent pulmonary hypertension of the newborn: safety issues relating to drug administration and delivery devices," DOVPress, vol. 6, no. 9, pp. 45-51, 2016.
[6] McGinn K, Reichert M, "A Comparison of Inhaled Nitric Oxide Versus Inhaled Epoprostenol for Acute Pulmonary Hypertension Following Cardiac Surgery.," Ann Pharmacother., vol. 50, no. 1, pp. 22-26, 2016.
[7] Chotigeat U, Champrasert M, Khorana M, Sangtaweesin V, Kanjanapattanakul W., "Iloprost inhalation for the treatment of severe persistent pulmonary hypertension of the newborn, experience at QSNICH.," J Med Assoc Thai., vol. 97, no. 6, pp. 89-94, 2014.
[8] Elkiran O, Karakurt C, Koçak G., "Combined effect of aerosolized iloprost and oxygen on assessment of pulmonary vasoreactivity in children with pulmonary hypertension.," Anadolu Kardiyol Derg, vol. 14, no. 4, pp. 383-388, 2014.
[9] A. Peliowski, "Inhaled nitric oxide use in newborns," Canadian Paediatric Society, 2017.
[10] Frederick E. Barr, Duncan Macrae, "Inhaled Nitric Oxide and Related Therapies," Pediatr Crit Care Med, vol. 11, no. 2, p. S30–S36, 2010.
[11] Afshari A, Brok J, Møller AM, Wetterslev J., "Inhaled nitric oxide for acute respiratory distress syndrome and acute lung injury in adults and children: a systematic review with meta-analysis and trial sequential analysis.," Anesth Analg., vol. 112, no. 6, pp. 1411-1421, 2011.
[12] Fernandes JL1, Sampaio RO, Brandão CM, Accorsi TA, Cardoso LF, Spina GS, Tarasoutchi F, Pomerantzeff P, Auler JO Jr, Grinberg M., "Comparison of inhaled nitric oxide versus oxygen on hemodynamics in patients with mitral stenosis and severe pulmonary hypertension after mitral valve surgery.," Am J Cardiol, vol. 107, no. 7, 2011.
[13] Kline JA. Et al, "Pilot study of a protocol to administer inhaled nitric oxide to treat severe acute submassive pulmonary embolism.," Emerg Med J, vol. 31, no. 6, pp. 459-462, 2014.
[14] Kallet RH, "Adjunct therapies during mechanical ventilation: airway clearance techniques, therapeutic aerosols, and gases.," Respir Care. , vol. 58, no. 6, pp. 1053-73, 2013.
[15] Gebistorf F1, Karam O, Wetterslev J, Afshari A., "Inhaled nitric oxide therapy for acute respiratory distress syndrome in children.," Cochrane Database Syst Rev. , vol. 27, no. 6, 2016.
[16] Chotigeat U, Champrasert M, Khorana M, Sangtaweesin V, Kanjanapattanakul W., "Iloprost inhalation for the treatment of severe persistent pulmonary hypertension of the newborn, experience at QSNICH.," J Med Assoc Thai., vol. 97, no. 6, pp. 89-94, 2014.
[17] THE NOBEL PRIZE, "The Nobel Prize in Physiology or Medicine 1998," The Nobel Prize Press release, 1998.
[17] Elkiran O, Karakurt C, Koçak G., "Combined effect of aerosolized iloprost and oxygen on assessment of pulmonary vasoreactivity in children with pulmonary hypertension.," Anadolu Kardiyol Derg, vol. 14, no. 4, pp. 383-388, 2014.
[18] G G Konduri, et al, for the Neonatal Inhaled Nitric Oxide Study Group, "Impact of early surfactant and inhaled nitric oxide therapies on outcomes in term/late preterm neonates with moderate hypoxic respiratory failure.," J Perinatol., vol. 33, no. 12, pp. 944-949, 2013.
[19] Durrani NU1, Chedid F, Rahmani A., "Neurally adjusted ventilatory assist mode used in congenital diaphragmatic hernia.," J Coll Physicians Surg Pak. , vol. 21, no. 10, pp. 637-9, 2011.
[20] DiBlasi RM1, Dupras D2, Kearney C2, Costa E Jr3, Griebel JL3., "Nitric Oxide Delivery by Neonatal Noninvasive Respiratory Support Devices.," Respir Care., vol. 60, no. 2, pp. 219-30, 2015.
[21] Qi Y, et al, "Circulating endothelial progenitor cells decrease in infants with bronchopulmonary dysplasia and increase after inhaled nitric oxide.," PLoS One. , vol. 8, no. 11, 2013.
[22] Ankita Das, "What is a difference between vasodilation and vasodilatation?," Quora, 2017.
[23] Breuer J, et al, "Technical considerations for inhaled nitric oxide therapy: time response to nitric oxide dosing changes and formation of nitrogen dioxide.," Eur J Pediatr., vol. 156, no. 6, pp. 460-462, 1997.
[24] Khorana M1, Yookaseam T, Layangool T, Kanjanapattanakul W, Paradeevisut H., "Outcome of oral sildenafil therapy on persistent pulmonary hypertension of the newborn at Queen Sirikit National Institute of Child Health.," J Med Assoc Thai. , pp. 64-73, 2011.
[25] Ammar MA1, Bauer SR2, Bass SN2, Sasidhar M2, Mullin R2, Lam SW2., "Noninferiority of Inhaled Epoprostenol to Inhaled Nitric Oxide for the Treatment of ARDS.," Ann Pharmacother., vol. 49, no. 10, pp. 1105-12, 2015.
[26] Elkiran O1, Karakurt C, Koçak G., "Combined effect of aerosolized iloprost and oxygen on assessment of pulmonary vasoreactivity in children with pulmonary hypertension.," Anadolu Kardiyol Derg. , vol. 14, no. 4, pp. 383-8, 2014.
[27] Rizza A1, Bignami E2, Belletti A2, Polito A3, Ricci Z3, Isgrò G4, Locatelli A5, Cogo P3., "Vasoactive Drugs and Hemodynamic Monitoring in Pediatric Cardiac Intensive Care: An Italian Survey.," World J Pediatr Congenit Heart Surg. , vol. 7, no. 1, pp. 25-31, 2016.
[28] Vakrilova L, Radulova P, Hitrova S, Slancheva B., "Pulmonary hypertension of the newborn--recent advances in the management and treatment," Akush Ginekol (Sofiia). , vol. 53, no. 4, pp. 50-8, 2014.
[29] MJ Mebius, EA Verhagen, ME van der Laan, AF Bos, "Cerebral Oxygen Saturation and Extraction in Neonates with Persistent Pulmonary Hypertension During the First 72 Hours of Life.," BMJ, vol. 97, no. 2, 2014.
[30] Robert M DiBlasi RRT-NPS FAARC, Timothy R Myers RRT-NPS, and Dean R Hess PhD RRT FAARC, "Evidence-Based Clinical Practice Guideline: Inhaled Nitric Oxide for Neonates With Acute Hypoxic Respiratory Failure," RESPIRATORY CARE, vol. 55, no. 12, 2010.


