SFDA Detects 345 Facilities Violating Food and Drug Regulations During January
2022-02-17
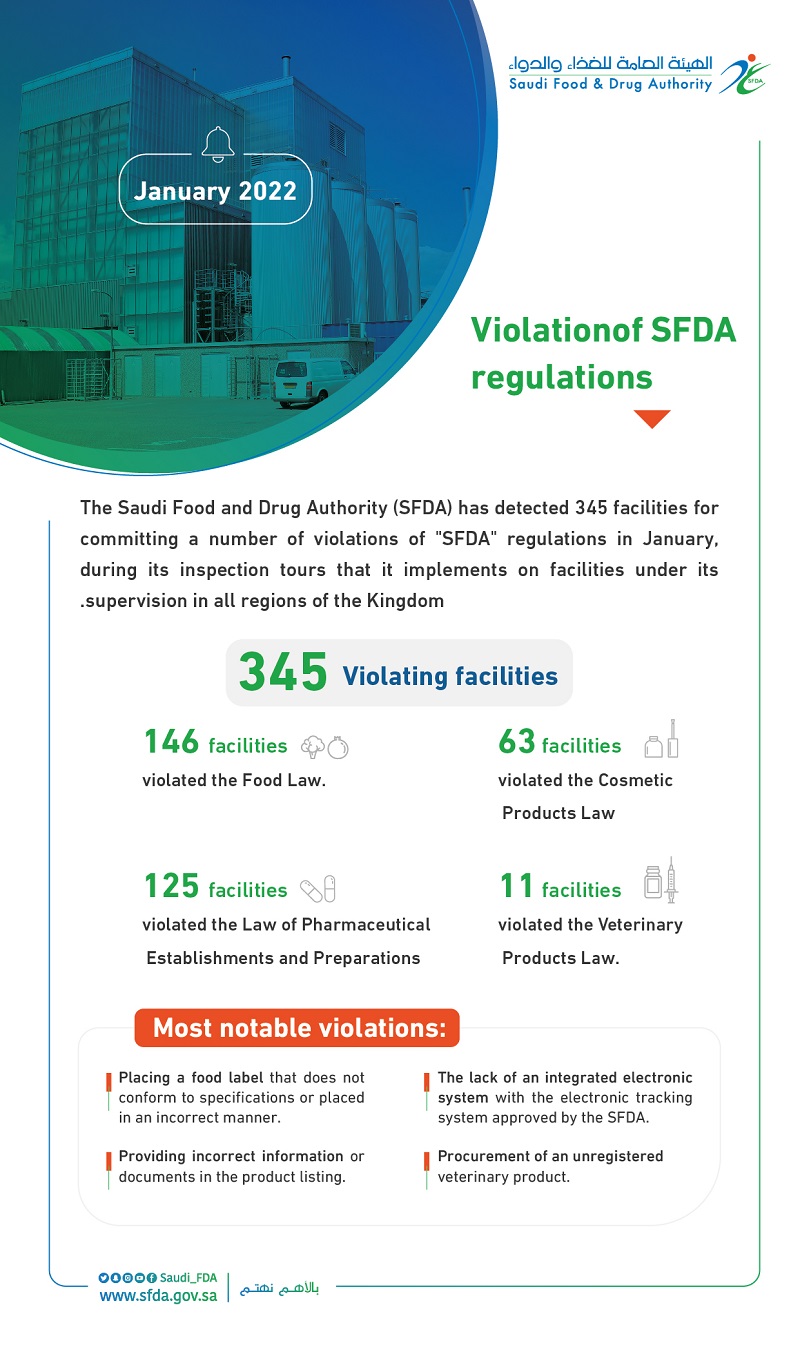
The Saudi Food and Drug Authority (SFDA) has detected 345 facilities for committing a number of violations of "SFDA" regulations in January, during its inspection tours that it implements on facilities under its supervision in all regions of the Kingdom.
SFDA pointed out that it has detected 146 facilities for violating the Food Law, and the violations include the placement of food label that did not conform to specifications or placed in an incorrect manner. According to the Food Law and its executive regulations, the penalty for this violation may reach one million riyals, in addition to preventing the violator from practicing any food business for a period of up to 180 days, and/or canceling the license and/or suspending the license for a period not exceeding one year.
SFDA also detected 125 pharmaceutical facilities due to the lack of an integrated electronic system with the electronic tracking system approved by the SFDA. According to the Law of Pharmaceutical Establishments and Preparations, the penalty for this violation may reach five million riyals, in addition to closing the pharmaceutical facility temporarily for a period not exceeding 180 days and/or canceling the license.
SFDA detected 63 facilities in violation of the Cosmetic Products Law. The violation was the submission of incorrect information or documents to the listing of the product, and according to the Cosmetic Products Law and its executive regulations, the penalty for this violation may reach five million riyals, in addition to closing the factory or warehouse until the violation is corrected and/or canceling the factory or warehouse license.
The inspection tours also included veterinary facilities, as a number of violations of the Veterinary Products Law were detected in 11 facilities. The violations were the procurement of an unregistered veterinary product, and the second article of the Veterinary Products Law states that “It is not permissible to import, market or trade any veterinary product unless it is registered with the competent authorities in the country”. The penalty for such violation may reach five million riyals, in addition to closing the factory or warehouse until the violation is corrected and/or canceling the factory or warehouse license, in accordance with the Veterinary Products Law and its executive regulations.