Post-Market Evaluation for the Safety and Effectiveness of Transvaginal Mesh for the Treatment of Pelvic Organ Prolapse (POP)
Post-Market Evaluation for the Safety and Effectiveness of Transvaginal Mesh for the Treatment of Pelvic Organ Prolapse (POP)
The safety and effectiveness of surgical mesh for transvaginal repair of pelvic organ prolapse have been questioned recently by numerous international regulatory offices and specialized societies, due to the accumulated risks revealed by medical devices reports and recently published studies [1]. Upon that, these products were stopped from distribution in the US [2] , Australia [3], Canada [4] , and the UK [56][57].
In this review, the safety and effectiveness of polypropylene transvaginal mesh products whose sole use is the treatment of pelvic organ prolapse (POP) via transvaginal route will be evaluated, with the purpose of deriving current-evidence recommendations to better regulate these products for protecting the patients safety.
Pelvic Organ Prolapse (POP)
The organs of the women pelvis (uterus, bladder, and rectum) are supported by muscles, known as the pelvic floor, as shown in figure 1A, which depicts the normal pelvic anatomy. Pelvic organ prolapse is a condition that happens when the muscles are weakened and no longer hold the pelvis organs in their normal places, which result in drops (prolapse) of these organs into the vagina [5].
There are different types of prolapse, which are classified in reference to which organ is dropping (bulging). Cystocele, or anterior wall prolapse, occurs a bladder drops from its normal position, as illustrated in figure 1B, while rectocele –posterior wall prolapse- refers to the drop of the rectum, figure 1C. Another prolapse type refers to the drop of the uterus into the vagina, which is known as uterine prolapse or procidentia, figure 1D. Also, it is common that more than one organ at the same time [5].
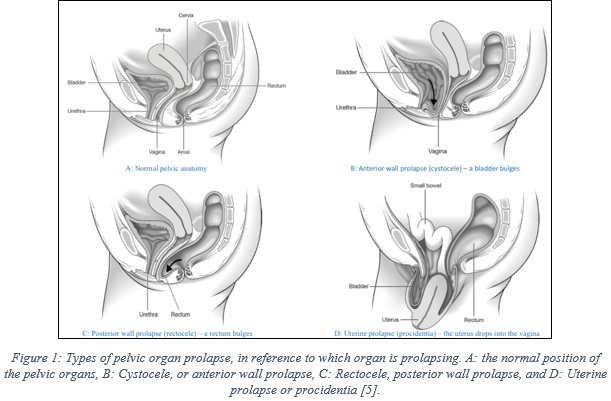
Pelvic organ prolapse is a common medical condition in women, with a prevalence rate of 41-50% of women, as reported in the US FDA report [1]. Patient age and obesity are major risk factors, which were reported to be associated with increased risk [6], beside other factors, such as, previous vaginal delivery, sexual activity, family history, and ethnicity [6].
Treatment options for POP
Pelvic organ prolapse can be treated in multiple ways, depending on the stage and type of the prolapse beside the patient characteristics. Treatment can be done either surgically or non-surgically. Non-surgical options involve using pessary -a plastic device that fits into the vagina to support the pelvic organs-, physiotherapy –special training to strengthen the pelvic muscles-, or by a medication –oestrogen therapy [6]. On the other hand, surgical options can be performed transabdominally –known as sacrocolpopexy-, or transvaginally, where both options aim to surgically correct the prolapsed organ [6].
Transvaginal surgical repair is used commonly to correct the anterior wall prolapse – figure 1B, which will be the focus of this review. Transvaginal repair can be done through two ways; either by using tissue and suture alone in a procedure known as anterior colporrhaphy -native tissue repair-, or through using surgical mesh, i.e. polypropylene, to augment the prolapsed organ [1] [5] [6]. Polypropylene mesh is a thin sheet of material, which can be in a non-configured form or a pre-configured form with legs to aid fixing the mesh into the desired area, as shown in figure 2. [1] [6]. The mesh can either be used alone, or with a mesh kits that facilitate the insertion and placement of the mesh.
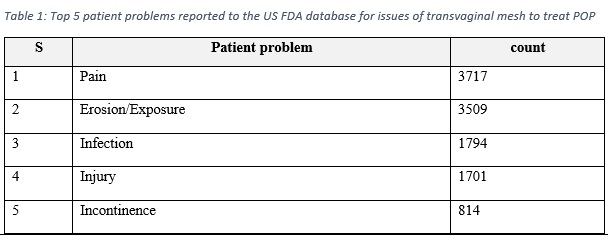
Medical devices reports provide real world evidence that aid in guiding the attention to the device risks. The US FDA declared that in the period of 2008-2018, there were a total of 11,274 MDRs, all relate to surgical mesh placed transvaginally in the anterior compartment to treat POP [1]. These reports included 77 report of deaths, and around 10,000 serious injuries [1]. Table 1 demonstrate the most five patient problems reported to the US FDA database.
The safety and effectiveness of transvaginal mesh for the treatment of POP were evaluated considering two directions: a review of the recently published papers in the topic, and the feedbacks of Saudi related society and experts.
Part 1: Clinical paper review
1.1 An overview of the search criteria
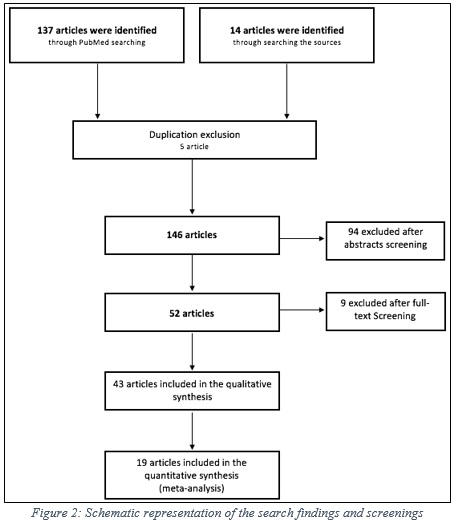
A systematic review and meta-analysis were conducted to compare the safety and effectiveness of transvaginal mesh and the alternative treatment option, native tissue repair. Using a defined inclusion criteria, a total of 151 articles were obtained and screened, which resulted in 43 articles for the quantitative analysis, and 19 articles (RCTs) for the meta-analysis, figure 2.
1.2 Results of calculating the mesh exposure weighted average
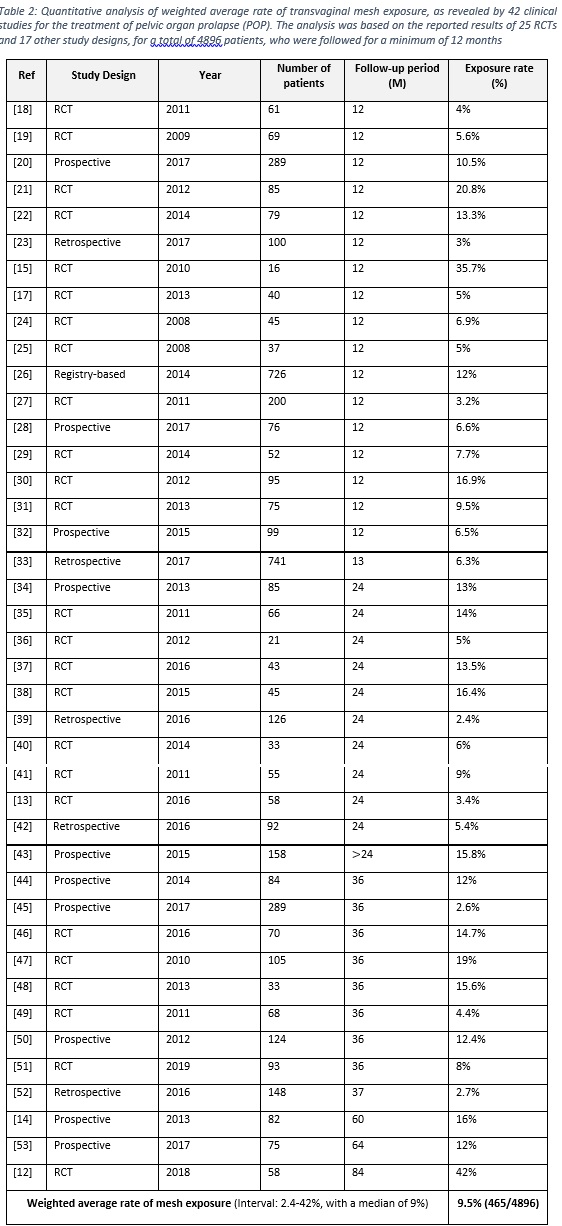
- A total of 41 articles (25 RCTs and 16 prospective and retrospective studies) were considered to calculate the weighted average of polypropylene mesh exposure. The total cases belong to 4896 patients, who were followed for at least 12 months.
- The weighted average of polypropylene mesh exposure was found to be 9.5% (465/4896), with an interval of 2.4-42% and a median of 9%, as illustrated in table 2.
- Mesh exposure was analyzed alone as only transvaginal repair by mesh is encounter of this risk, whereas women undergoing colporrhaphy have no risk of mesh exposure.
1.3 Results of comparing the risk of De novo dyspareunia when using TVM vs NTR
- Data of 15 RCTs were included in this analysis, which represent 854 patients in the mesh group, in contrast to 867 patients in the NTR group.
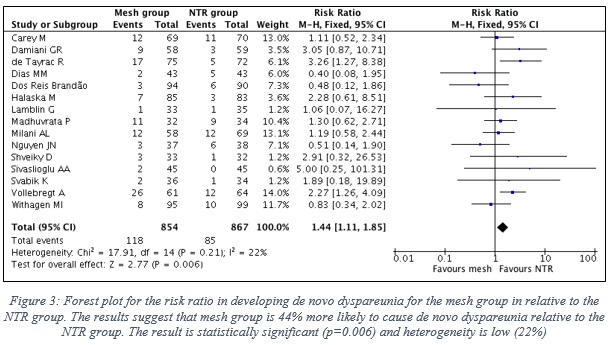
- Figure 3 shows the forest plot with the statistical analysis between the two groups.
- Pooled risk ratio (RR) is 1.44 in favor to NTR group (95% CI, 1.11-1.85), which clearly indicates that the mesh group is 44% more likely to cause de novo dyspareunia relative to the NTR group.
1.4 Results of comparing the risk of De SUI when using TVM vs NTR
- Data of 10 RCTs were included in this analysis, which represent 728 patients in the mesh group, in contrast to 725 patients in the NTR group.
- Figure 4 shows the forest plot with the statistical analysis between the two groups.
- Pooled risk ratio (RR) is 1.43 in favor to NTR group (95% CI, 1.10-1.87), which clearly indicates that the mesh group is 43% more likely to cause de novo SUI relative to the NTR group.
1.5 Results of the effectiveness of TVM vs NTR considering the prolapse recurrence
- Data of 10 RCTs were included in this analysis, which represent 740 patients in the mesh group, in contrast to 735 patients in the NTR group. Note: Prolapse recurrence of all types were included in this analysis.
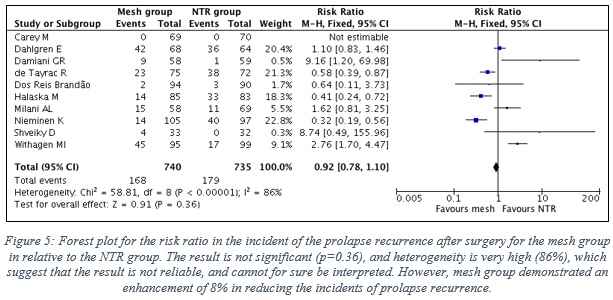
- Figure 5 shows the forest plot with the statistical analysis between the two groups.
- The result is not significant (p=0.36), and heterogeneity is very high (86%) between the results of the included studies, which suggest that the result is not reliable, and cannot for sure be interpreted. Yet, the pooled result suggest that mesh group demonstrated an enhancement of 8% in reducing the risk of prolapse recurrence relative to the NTR group.
1.6 Results of the effectiveness of TVM vs NTR considering the need for reoperation
- Data of 6 RCTs were included, which represent 590 patients in the mesh group, in contrast to 565 patients in the NTR group. Note: Surgery reoperation of all types were included in this analysis.
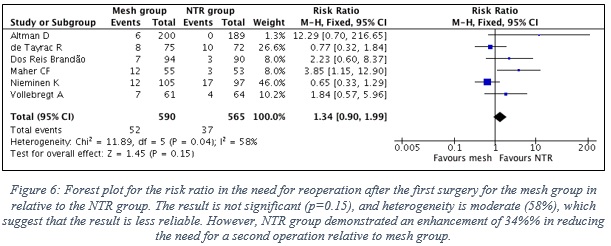
- Figure 6 shows the forest plot with the statistical analysis between the two groups.
- The result is not significant (p=0.15), and heterogeneity is moderate (58%) between the results of the included studies, which suggest that the result is moderately reliable, and cannot for sure be interpreted. Yet, mesh group is 34% more likely to require reoperation relative to NTR group.
1.7 Overall results
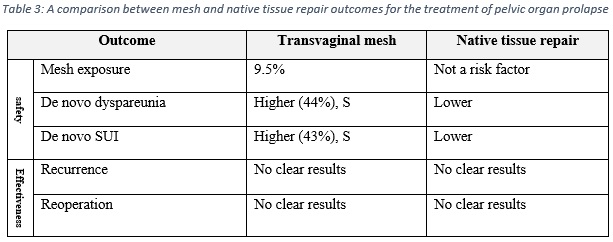
The overall results are summarized in table 3, which shows that the risks of using synthesis polypropylene mesh for the treatment of POP outweigh the benefits.
Part 2: Saudi user experience
2.1 The opinion of the Saudi Urological Association
In the 2nd of February, 2020, the SFDA team conducted a consultation meeting with the Saudi Urological Association (SUA), represented by Dr. Badr N. Almosaieed, to discuss the safety of transvaginal mesh for POP. Following the discussion, SUA officially submitted its position regarding the case, which indicate that these products carry risks that outweigh the benefits, and due to the availability of other safer treatment options, the society recommend suspending these products from the Saudi market, for the sake of the patients safety.
2.2 Device related incidents as provided by some Saudi users
Experts from the Saudi Voiding Dysfunction Group-Saudi Urological Association, beside other Saudi consultants, were asked to submit an evaluation assessment to clarify if they experienced any incidents with patients who were treated for POP using polypropylene mesh products. A number of 23 responses were received, with a reporting of 20 serious mesh-related incidents, which correspond to 9 mesh erosion cases, 9 dyspareunia cases, and 2 cases of organ perforation.
Part 3: Overall conclusion
Considering the results of the published papers, the recommendation of the Saudi Urological Association, and the incidents reported by the Saudi users, the overall evaluation suggests that the risk of using polypropylene mesh for the transvaginal repair of POP is outweighing the benefits.
Considering the results of the post-market evaluation of the safety and effectiveness of transvaginal mesh for the treatment of POP, the following actions were taken by SFDA:
- Suspending the marketing authorization of surgical mesh products whose sole use is the treatment of pelvic organ prolapse (POP) through transvaginal implantation.
- Stop authorizing new surgical mesh products whose sole use is the treatment of pelvic organ prolapse (POP) through transvaginal implantation.
- Request a label change for synthetic surgical mesh devices indicated for the treatment of POP to include the warning: Do not use transvaginally.
- Review the technical file and the product instructions for use (IFU) of some polypropylene products that are used for other purposes, i.e. the treatment of hernia, to ensure that the products are not indicated for the treatment of POP through transvaginal implantation, otherwise, the products are to be treated as indicated in recommendation number 3.
Grateful thanks to Eng. Bader Aloufi for designing, reviewing the up to date articles, and writing up the context of this study. Thanks to the post-market clinical evaluation team for their supports in conducting this work.
For further information or inquiries related to this study, you may contact us at: cia.md@sfda.gov.sa
[1]
US FDA, "Surgical Mesh for Transvaginal Repair of Pelvic Organ Prolapse in the Anterior Vaginal Compartment," US FDA, 2019.
[2]
US FDA, "Urogynecologic Surgical Mesh Implants," 10 07 2019. [Online]. Available: https://www.fda.gov/medical-devices/implants-and-prosthetics/urogynecologic-surgical-mesh-implants. [Accessed 20 12 2019].
[3]
"TGA actions after review into urogynaecological surgical mesh implants," 17 05 2019. [Online]. Available: https://www.tga.gov.au/alert/tga-actions-after-review-urogynaecological-surgical-mesh-implants. [Accessed 20 12 2019].
[4]
Health Canada, "Status of non-absorbable synthetic surgical mesh for the transvaginal repair of pelvic organ prolapse in Canada," 26 07 2019. [Online]. Available: https://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2019/70563a-eng.php. [Accessed 20 12 2019].
[5]
Royal College of Obstetricians & Gynaecologists, "Pelvic organ prolapse," Royal College of Obstetricians and Gynaecologists, 2013.
[6]
Scientific Committee on Emerging and Newly Identified Health Risks, "Opinion on The safety of surgical meshes used in urogynecological surgery," European Commission, 2015.
[7]
MarketWatch, "Global Transvaginal Mesh Market 2019 Share, Size Movements by Trend Analysis, Scope, Opportunities, Growth Status, Top Key Players, Revenue Expectation to 2026: Market Reports World," Market Watch, 2019.
[8]
KAKE, "Detailed Study on Transvaginal Mesh Industry 2019 by Size, Manufacturers, Demand, Applications and 2026 Forecast Research Report," KAKE: Original Research, 2019.
[9]
David Moher1,2*, Alessandro Liberati3,4, Jennifer Tetzlaff1, Douglas G. Altman5, The PRISMA Group", "Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement," PLoS Medicine, vol. 9, no. 7, 2009.
[10]
Christopher Maher, Benjamin Feiner, Kaven Baessler, Corina Christmann‐Schmid, Nir Haya, Jane Marjoribanks, "Transvaginal mesh or grafts compared with native tissue repair for vaginal prolapse," Cochrane Library, 2016.
[11]
C. de Vries et al., "Long-term complications of transvaginal mesh implants A literature review," National Institute of public health, Netherland, 2018.
[12]
Milani AL1, Damoiseaux A2, IntHout J3, Kluivers KB4, Withagen MIJ5., "Long-term outcome of vaginal mesh or native tissue in recurrent prolapse: a randomized controlled trial.," Int Urogynecol J. , vol. 29, no. 6, pp. 847-858, 2018.
[13]
Damiani GR1,2,3,4, Riva D5, Pellegrino A1, Gaetani M2, Tafuri S6, Turoli D1, Croce P7, Loverro G2., "Conventional fascial technique versus mesh repair for advanced pelvic organ prolapse: Analysis of recurrences in treated and untreated compartments.," J Obstet Gynaecol. , vol. 36, no. 3, pp. 410-5, 2016.
[14]
Jacquetin B1, Hinoul P, Gauld J, Fatton B, Rosenthal C, Clavé H, Garbin O, Berrocal J, Villet R, Salet-Lizée D, Debodinance P, Cosson M., "Total transvaginal mesh (TVM) technique for treatment of pelvic organ prolapse: a 5-year prospective follow-up study.," Int Urogynecol J. , vol. 24, no. 10, pp. 1679-86, 2013.
[15]
Lopes ED1, Lemos NL, Carramão Sda S, Lunardelli JL, Ruano JM, Aoki T, Auge AP., "Transvaginal polypropylene mesh versus sacrospinous ligament fixation for the treatment of uterine prolapse: 1-year follow-up of a randomized controlled trial," Int Urogynecol J. , vol. 21, no. 4, pp. 389-94, 2010.
[16]
Dos Reis Brandão da Silveira S1, Haddad JM, de Jármy-Di Bella ZI, Nastri F, Kawabata MG, da Silva Carramão S, Rodrigues CA, Baracat EC, Auge AP., "Multicenter, randomized trial comparing native vaginal tissue repair and synthetic mesh repair for genital prolapse surgical treatment.," Int Urogynecol J., vol. 26, no. 3, pp. 335-42, 2015.
[17]
Delroy CA1, Castro Rde A, Dias MM, Feldner PC Jr, Bortolini MA, Girão MJ, Sartori MG., "The use of transvaginal synthetic mesh for anterior vaginal wall prolapse repair: a randomized controlled trial.," Int Urogynecol J. , vol. 24, no. 11, pp. 1899-907, 2013.
[18]
Vollebregt A1, Fischer K, Gietelink D, van der Vaart CH., "Primary surgical repair of anterior vaginal prolapse: a randomised trial comparing anatomical and functional outcome between anterior colporrhaphy and trocar-guided transobturator anterior mesh.," BJOG., vol. 118, no. 12, pp. 1518-27, 2011.
[19]
Carey M1, Higgs P, Goh J, Lim J, Leong A, Krause H, Cornish A., "Vaginal repair with mesh versus colporrhaphy for prolapse: a randomised controlled trial.," BJOG., vol. 116, no. 10, pp. 1380-6, 2009.
[20]
Fünfgeld C1, Stehle M1, Henne B2, Kaufhold J3, Watermann D4, Grebe M5, Mengel M6., "Quality of Life, Sexuality, Anatomical Results and Side-effects of Implantation of an Alloplastic Mesh for Cystocele Correction at Follow-up after 36 Months.," Geburtshilfe Frauenheilkd., vol. 77, no. 9, pp. 993-1001, 2017.
[21]
Halaska M1, Maxova K, Sottner O, Svabik K, Mlcoch M, Kolarik D, Mala I, Krofta L, Halaska MJ., "A multicenter, randomized, prospective, controlled study comparing sacrospinous fixation and transvaginal mesh in the treatment of posthysterectomy vaginal vault prolapse.," Am J Obstet Gynecol., vol. 207, no. 4, pp. 301.e1-7, 2012.
[22]
Rudnicki M1, Laurikainen E, Pogosean R, Kinne I, Jakobsson U, Teleman P., "Anterior colporrhaphy compared with collagen-coated transvaginal mesh for anterior vaginal wall prolapse: a randomised controlled trial.," BJOG., vol. 121, no. 1, pp. 102-10, 2014.
[23]
Barros-Pereira I1, Valentim-Lourenço A1, Fonseca A1, Melo B2, Henriques A1, Ribeirinho A1., "A retrospective analysis of the effectiveness of anterior pelvic organ prolapse repair with Prolift versus Elevate vaginal mesh.," Int J Gynaecol Obstet., vol. 139, no. 2, pp. 192-196, 2017.
[24]
Sivaslioglu AA1, Unlubilgin E, Dolen I., "A randomized comparison of polypropylene mesh surgery with site-specific surgery in the treatment of cystocoele.," Int Urogynecol J Pelvic Floor Dysfunct., vol. 19, no. 4, pp. 467-71, 2008.
[25]
Nguyen JN1, Burchette RJ., "Outcome after anterior vaginal prolapse repair: a randomized controlled trial.," Obstet Gynecol. , vol. 111, no. 4, pp. 891-8, 2008.
[26]
Bjelic-Radisic V1, Aigmueller T, Preyer O, Ralph G, Geiss I, Müller G, Riss P, Klug P, Konrad M, Wagner G, Medl M, Umek W, Lozano P, Tamussino K, Tammaa A; Austrian Urogynecology Working Group., "Vaginal prolapse surgery with transvaginal mesh: results of the Austrian registry.," Int Urogynecol J., vol. 25, no. 8, pp. 1047-52, 2014.
[27]
Altman D1, Väyrynen T, Engh ME, Axelsen S, Falconer C; Nordic Transvaginal Mesh Group., "Anterior colporrhaphy versus transvaginal mesh for pelvic-organ prolapse.," N Engl J Med. , vol. 364, no. 19, pp. 1826-36, 2011.
[28]
Gutman RE1, Rardin CR2, Sokol ER3, Matthews C4, Park AJ5, Iglesia CB5, Geoffrion R6, Sokol AI5, Karram M7, Cundiff GW6, Blomquist JL8, Barber MD9., "Vaginal and laparoscopic mesh hysteropexy for uterovaginal prolapse: a parallel cohort study.," Am J Obstet Gynecol. , vol. 216, no. 1, pp. e1-38.e11, 2017.
[29]
B Gupta, MD; N B Vaid, MD; A Suneja, MD; K Guleria, MD; S Jain, MD, "Anterior vaginal prolapse repair: A randomised trial of traditional anterior colporrhaphy and self-tailored mesh repair," SAJOG, vol. 20, no. 2, pp. 47-50, 2014.
[30]
Withagen MI1, Milani AL, de Leeuw JW, Vierhout ME., "Development of de novo prolapse in untreated vaginal compartments after prolapse repair with and without mesh: a secondary analysis of a randomised controlled trial.," BJOG. , vol. 119, no. 3, pp. 354-60, 2012.
[31]
de Tayrac R1, Cornille A, Eglin G, Guilbaud O, Mansoor A, Alonso S, Fernandez H., "Comparison between trans-obturator trans-vaginal mesh and traditional anterior colporrhaphy in the treatment of anterior vaginal wall prolapse: results of a French RCT.," Int Urogynecol J., vol. 24, no. 10, pp. 1651-61, 2013.
[32]
Jirschele K1, Seitz M, Zhou Y, Rosenblatt P, Culligan P, Sand P., "A multicenter, prospective trial to evaluate mesh-augmented sacrospinous hysteropexy for uterovaginal prolapse.," Int Urogynecol J. , vol. 26, no. 5, pp. 743-8, 2015.
[33]
Cheng YW1, Su TH2, Wang H1, Huang WC3, Lau HH4., "Risk factors and management of vaginal mesh erosion after pelvicorgan prolapse surgery," Taiwan J Obstet Gynecol. , vol. 56, no. 2, pp. 184-187, 2017.
[34]
Marianna Alperin, MD, MS,* Rennique Ellison, BS,* Leslie Meyn, MS,† Elizabeth Frankman, MD, MS,* and Halina M. Zyczynski, MD*, "Two-Year Outcomes After Vaginal Prolapse Reconstruction With Mesh Pelvic Floor Repair System," Female Pelvic Med Reconstr Surg. , vol. 19, no. 2, pp. 72-78, 2013.
[35]
Menefee SA1, Dyer KY, Lukacz ES, Simsiman AJ, Luber KM, Nguyen JN., "Colporrhaphy compared with mesh or graft-reinforced vaginal paravaginal repair for anterior vaginal wall prolapse: a randomized controlled trial," Obstet Gynecol. , vol. 118, no. 6, pp. 1337-44, 2011.
[36]
El-Nazer MA1, Gomaa IA, Ismail Madkour WA, Swidan KH, El-Etriby MA., "Anterior colporrhaphy versus repair with mesh for anterior vaginal wall prolapse: a comparative clinical study," Arch Gynecol Obstet. , vol. 286, no. 4, pp. 965-72, 2012.
[37]
Dias MM1, de A Castro R1, Bortolini MA1, Delroy CA1, Martins PC1, Girão MJ1, Sartori MG1., "Two-years results of native tissue versus vaginal mesh repair in the treatment of anterior prolapse according to different success criteria: A randomized controlled trial.," Neurourol Urodyn. , vol. 35, no. 4, pp. 509-14, 2016.
[38]
Tamanini JT1, de Oliveira Souza Castro RC2, Tamanini JM3, Castro RA4, Sartori MG4, Girão MJ4., "A prospective, randomized, controlled trial of the treatment of anterior vaginal wall prolapse: medium term followup.," J Urol. , vol. 193, no. 4, pp. 1298-304, 2015.
[39]
Lamblin G1, Gouttenoire C2, Panel L2, Moret S3, Chene G3, Courtieu C2., "A retrospective comparison of two vaginal mesh kits in the management of anterior and apical vaginal prolapse: long-term results for apical fixation and quality of life.," Int Urogynecol J., vol. 27, no. 12, pp. 1847-1855, 2016.
[40]
Lamblin G1, Van-Nieuwenhuyse A, Chabert P, Lebail-Carval K, Moret S, Mellier G., "A randomized controlled trial comparing anatomical and functional outcome between vaginal colposuspension and transvaginal mesh.," Int Urogynecol J., vol. 25, no. 7, pp. 961-70, 2014.
[41]
Maher CF1, Feiner B, DeCuyper EM, Nichlos CJ, Hickey KV, O'Rourke P., "Laparoscopic sacral colpopexy versus total vaginal mesh for vaginal vault prolapse: a randomized trial.," Am J Obstet Gynecol. , vol. 204, no. 4, pp. 360.e1-7, 2011.
[42]
Wu PY1, Chang CH2, Shen MR1, Chou CY1, Yang YC3, Huang YF4., "Seeking new surgical predictors of mesh exposure after transvaginal mesh repair.," Int Urogynecol J., vol. 27, no. 10, pp. 1547-55, 2016.
[43]
Karmakar D1, Hayward L, Smalldridge J, Lin S., "Vaginal mesh for prolapse: a long-term prospective study of 218 mesh kits from a single centre.," Int Urogynecol J., vol. 26, no. 8, pp. 1161-70, 2015.
[44]
Bontje HF1, van de Pol G, van der Zaag-Loonen HJ, Spaans WA., "Follow-up of mesh complications using the IUGA/ICS category-time-site coding classification.," Int Urogynecol J. , vol. 25, no. 6, pp. 817-22, 2014.
[45]
Fünfgeld C1, Stehle M1, Henne B2, Kaufhold J3, Watermann D4, Grebe M5, Mengel M6., "Quality of Life, Sexuality, Anatomical Results and Side-effects of Implantation of an Alloplastic Mesh for Cystocele Correction at Follow-up after 36 Months.," Geburtshilfe Frauenheilkd. , vol. 77, no. 9, pp. 993-1001, 2017.
[46]
Rudnicki M1, Laurikainen E2, Pogosean R3, Kinne I4, Jakobsson U5, Teleman P6., "A 3-year follow-up after anterior colporrhaphy compared with collagen-coated transvaginal mesh for anterior vaginal wall prolapse: a randomised controlled trial.," BJOG. , vol. 123, no. 1, pp. 136-42, 2016.
[47]
Nieminen K1, Hiltunen R, Takala T, Heiskanen E, Merikari M, Niemi K, Heinonen PK., "Outcomes after anterior vaginal wall repair with mesh: a randomized, controlled trial with a 3 year follow-up.," Am J Obstet Gynecol. , vol. 203, no. 3, pp. 235.e1-8, 2010.
[48]
Shveiky D1, Sokol AI, Gutman RE, Kudish BI, Iglesia CB., "Patient goal attainment in vaginal prolapse repair with and without mesh.," Int Urogynecol J. , vol. 23, no. 11, pp. 1541-6, 2012.
[49]
Dahlgren E1, Kjølhede P; RPOP-PELVICOL Study Group., "Long-term outcome of porcine skin graft in surgical treatment of recurrent pelvic organ prolapse. An open randomized controlled multicenter study.," Acta Obstet Gynecol Scand. , vol. 90, no. 12, pp. 1393-401, 2011.
[50]
Long CY1, Hsu CS, Wu CH, Liu CM, Wang CL, Tsai EM., "Three-year outcome of transvaginal mesh repair for the treatment of pelvic organ prolapse.," Eur J Obstet Gynecol Reprod Biol. , vol. 161, no. 1, pp. 105-8, 2012.
[51]
Nager CW1, Visco AG2, Richter HE3, Rardin CR4, Rogers RG5,6, Harvie HS7, Zyczynski HM8, Paraiso MFR9, Mazloomdoost D10, Grey S11, Sridhar A11, Wallace D11; NICHD Pelvic Floor Disorders Network., "Effect of Vaginal Mesh Hysteropexy vs Vaginal Hysterectomy With Uterosacral Ligament Suspension on Treatment Failure in Women With Uterovaginal Prolapse: A Randomized Clinical Trial.," JAMA. , vol. 322, no. 11, pp. 1054-1065, 2019.
[52]
Hüsch T1, Mager R2, Ober E3, Bentler R3, Ulm K4, Haferkamp A2., "Quality of life in women of non-reproductive age with transvaginal mesh repair for pelvic organ prolapse: A cohort study.," Int J Surg. , vol. 33, pp. A:36-41, 2016.
[53]
Laso-García IM1, Rodríguez-Cabello MA2, Jiménez-Cidre MA2, Orosa-Andrada A2, Carracedo-Calvo D2, López-Fando L2, Burgos-Revilla FJ2., "Prospective long-term results, complications and risk factors in pelvic organ prolapse treatment with vaginal mesh.," Eur J Obstet Gynecol Reprod Biol. , vol. 211, pp. 62-67, 2017.
[54]
Svabik K1, Martan A, Masata J, El-Haddad R, Hubka P., "Comparison of vaginal mesh repair with sacrospinous vaginal colpopexy in the management of vaginal vault prolapse after hysterectomy in patients with levator ani avulsion: a randomized controlled trial.," Ultrasound Obstet Gynecol., vol. 43, no. 4, pp. 365-71, 2014.
[55]
Madhuvrata P1, Glazener C, Boachie C, Allahdin S, Bain C., "A randomised controlled trial evaluating the use of polyglactin (Vicryl) mesh, polydioxanone (PDS) or polyglactin (Vicryl) sutures for pelvic organ prolapse surgery: outcomes at 2 years.," J Obstet Gynaecol. , vol. 31, no. 5, pp. 429-35, 2011.
[56]
Medicines and Healthcare products Regulatory Agency, "Pause on the use of vaginally inserted surgical mesh for stress urinary incontinence," 2018. [Online]. Available: https://www.gov.uk/government/news/pause-on-the-use-of-vaginally-inserted-surgical-mesh- for-stress-urinary-incontinence.
[57]
Scottish Government, "Halt in use of transvaginal mesh," 2018. [Online]. Available: https://www.gov.scot/news/halt-in-use-of-transvaginal-mesh.


