General health
General health
Reduced effectiveness of Plavix (clopidogrel) in patients who are poor metabolizers of the drug
2010-03-23
The U.S. Food and Drug Administration (FDA), the counterpart for Saudi Food and Drug Authority (SFDA), has added a Boxed Warning to the label for Plavix, the anti-blood clotting medication. The Boxed Warning is about patients who do not effectively metabolize the drug (i.e. "poor metabolizers") and therefore may not receive the full benefits of the drug.
FDA Drug Safety Communication: Ongoing Safety Review of Stalevo (entacapone/carbidopa/levodopa) and possible development of Prostate Cancer
2010-05-17
The U.S. Food and Drug Administration (FDA) is evaluating clinical trial data that may suggest that patients taking Stalevo, a Parkinson's disease medication, may be at an increased risk for developing prostate cancer. In this trial, patients taking Stalevo were compared to those taking carbidopa and levodopa (sold as Sinemet), a combination medication also used to treat Parkinson's disease.
At this time, FDA's review of Stalevo is ongoing and no new conclusions or recommendations about the use of this drug have been made.
Safety Review of Proton Pump Inhibitors and Possible Increased Risk of Fracture of the Hip, Wrist, and Spine
2010-06-28
The Saudi Food and Drug Authority (SFDA) has revised the labels for a class of drugs known as proton pump inhibitors (PPIs) to include new safety information about a possible increased risk of fractures of the hip, wrist, and spine with the use of these medications at higher doses or long duration (more than one year).
Revision and Updating of Prescribing Information for Tramadol Containing Products
2010-07-03
The Saudi Food and Drug Authority (SFDA) notifies healthcare professionals of changes to the Warnings section of the prescribing information for tramadol, a centrally acting synthetic opioid analgesic indicated for the management of moderate to moderately severe chronic pain. The strengthened Warnings information was based on several reports Tramadol-related deaths have occurred in patients with previous histories of emotional disturbances or suicidal ideation or attempts, as well as histories of misuse of tranquilizers, alcohol, and other CNS-active drugs.
B-VITAMINS AND DIABETIC NEPHROPATHY
2010-08-07
The Journal of the American Medical Association published a randomized controlled trial on April 28, 2010 that found the administration of B-vitamins failed to slow the progression of diabetic nephropathy and was associated with an increase in vascular events. The trial was stopped early for safety reasons.1
SFDA warns about Purchasing Weight-Reducing Products through Media and Social Communication Systems
2013-01-03
In view of its role of monitoring and control and in implementation of its mission of ensuring safety , quality and efficacy of drugs & safety of health products; SFDA observed that several health and herbal products are being illegally marketed through TV Channels, SMS or Advertisements wherein consumers are attracted by the ability of such products to reduce high percentage of weight in a short period of time.
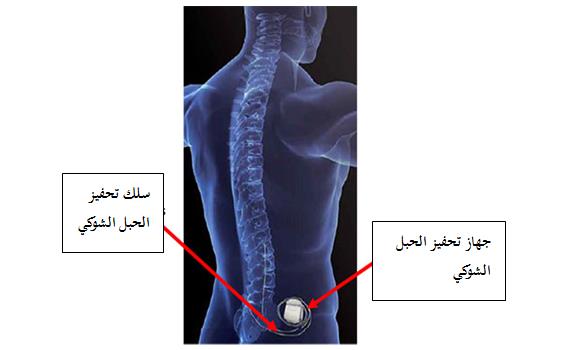
الهيئة تنبه من المخاطر المحتملة نتيجة تكوّن كتل من الأنسجة حول أسلاك أجهزة تحفيز الحبل الشوكي المزروعة للمرضى والمصنعة بواسطة شركة ميدترونك الطبية Medtronic
2014-07-14
تود الهيئة العامة للغذاء والدواء أن تنبه من المخاطر المحتملة المتعلقة بالمرضى الذين أجريت لهم عمليات زراعة أجهزة تحفيز الحبل الشوكي المصنعة من قبل شركة مدترونيك الطبية )انظر إلى القائمة أدناه لأجهزة التحفيز والطرازات والمجموعات وأرقامها المتأثرة بالتحذير (،فمن المحتمل تعرضهم لمخاطر وبنسبة منخفضة لتكوّن كتل من الأنسجة حول أسلاك الجهاز التي يمكن أن تسبب انضغاط الحبل الشوكي،و أجهزة تحفيز الحبل الشوكي هي أجهزة يتم زرعها تحت الجلد ويتم برمجتها لإرسال التحفيز الكهربائي للحبل الشوكي، وتستخدم للمساعدة في علاج أنواع معينة من الآلام المزمنة في الجذع و/أو الأطراف، والآلام المر
Saudi Food and Drug Authority alert about use of ophthalmic laser applications in eyes containing KAMRA™ inlays manufactured by AcuFocus Inc
2015-05-05
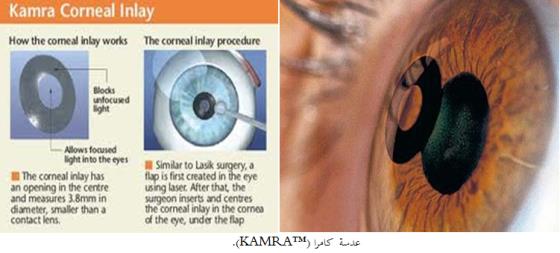
تود الهيئة العامة للغذاء والدواء أن تنبه جراحي وأطباء العيون وأخصائيي الليزر المستخدم لعلاج العين بخصوص التوصيات التي أصدرها مصنع أكوفوكس إنك (AcuFocus™ Inc) حول استخدام بعض تطبيقات الليزر للمرضى الذين زرعت لهم عدسات من العلامة التجارية كامرا(KAMRA™).
العدسات المزروعة من العلامة التجارية كامرا (KAMRA™) تستخدم لعلاج قصر النظر الشيخوخي، وهي عبارة عن قرص مجهري قطره 3.8 ملم وله فتحة في الوسط وهو أصغر بكثير من العدسات اللاصقة وأخف وزناً من حبة الملح.
Saudi Food and Drug Authority warns from using OneTouch® Verio® Pro+ blood glucose meters (Hospital and clinics use)
2013-05-23
Saudi Food and Drug Authority represented by the medical devices sector would like to warn all healthcare practitioners from using OneTouch® Verio® Pro+ blood glucose meters (hospital and clinics use), manufactured by Lifescan Johnson & Johnson company due to incorrect test results stored in results log at extremely high blood glucose levels above 1023 mg/dL (56.83 mmol/l).
SFDA has become aware through Lifescan Johnson & Johnson that recalling and replacing all OneTouch Verio Pro+ blood glucose meters because:
- Previous page
- Page 29
- Next page