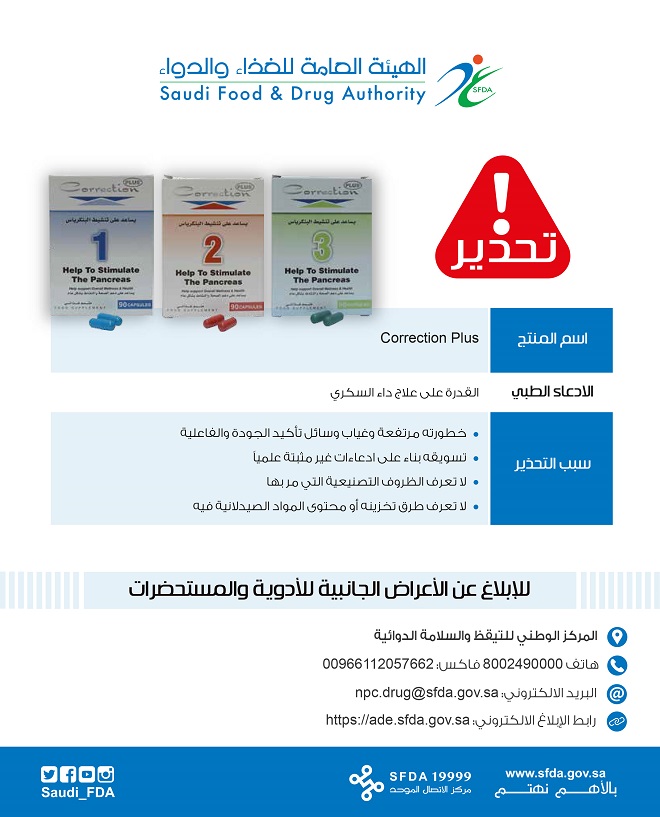
SFDA warns about ‘Correction Plus’ product as it conations medical claims in violation of diabetes treatments
2016-11-07
SFDA warned about ‘Correction Plus’ product that is promoted via a website claiming that it cures diabetes while it contains violating medical claims
In a statement, published on its website’ ’ , SFDA clarified that pursuant to its role of assurance of safety, quality and efficacy of drug and safety of pharmaceutical products that are marketed within the Kingdom, SFDA observed a website that promotes a product named ‘Correction Plus’ claiming that it cures diabetes and that it is a nutritional complement that does not require a marketing authorization from SFDA.
SFDA warned consumers from the danger of using this product due to its unknown manufacturing process or method of storage or content of pharmaceutical substances as well as the lack of the means of assurance of product safety, quality and efficacy, in addition to marketing it on the basis of unproven claims
SFDA advised consumers not to believe such misleading advertisements published on unlicensed marketing media that promotes drugs of unknown sources and that are not registered or released by SFDA, saying that it is coordinating with the respective authorities to chase such promoters and penalize them according to regulations.
Registered products can be viewed at SFDA website and registration number shown on the package can be verified therefrom
SFDA called on consumers to report any drug side effect to the National Pharmacovigilence Center on free phone number 8002490000 or Fax +966112057662 or universal number 19999 or e-mail or the following link: