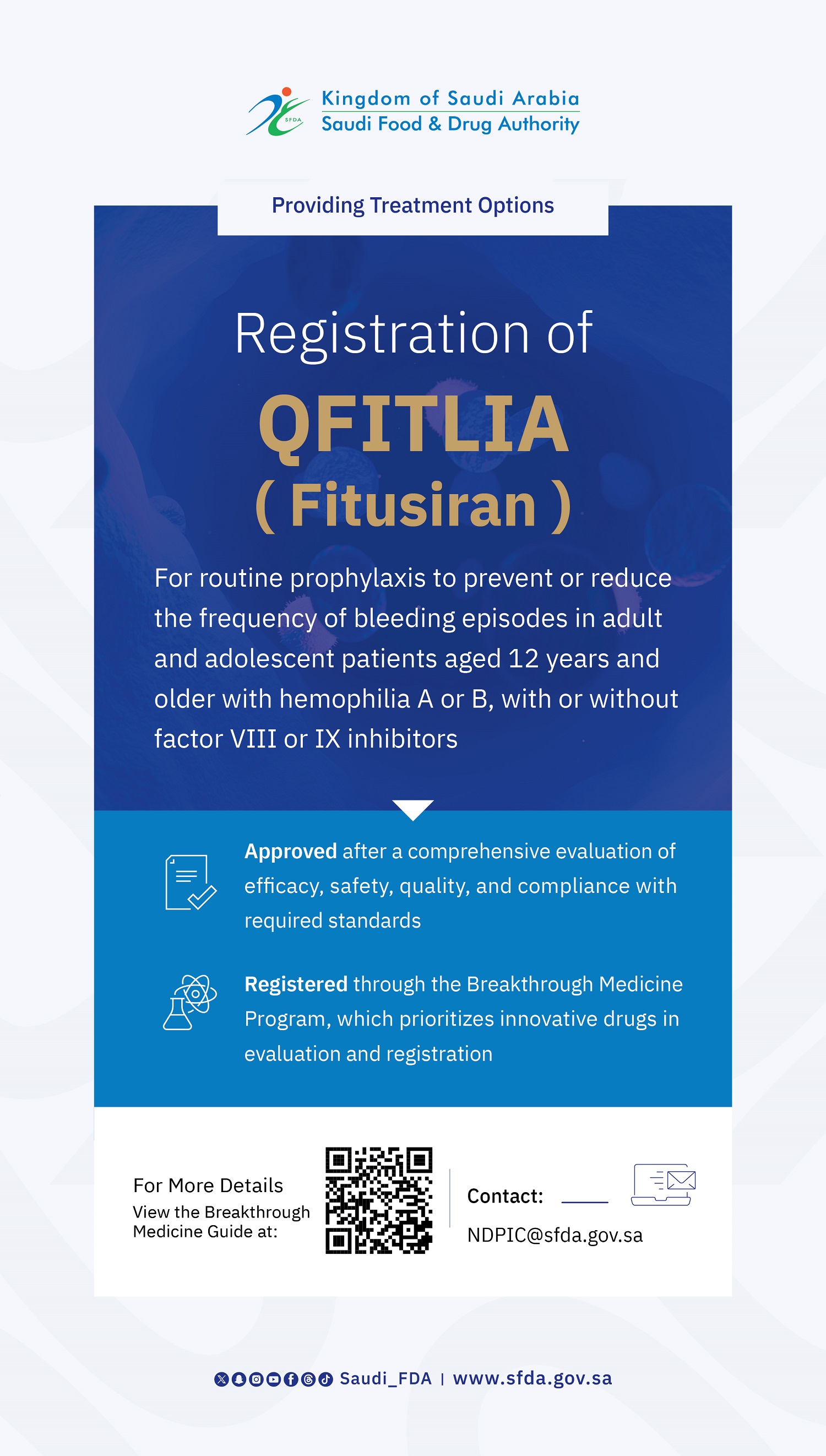
The SFDA Approves the Registration of Elrexfio for the Treatment of Adults with Multiple Myeloma
2025-08-12
The Saudi Food and Drug Authority (SFDA) has approved the registration of Elrexfio (Elranatamab) for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least four prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody. This Product has been granted an orphan drug designation under the SFDA Orphan Drug Program.
Elranatamab: A Bispecific Antibody
Elrexfio contains the active substance Elranatamab, which is a bispecific antibody that targets BCMA, a protein found on multiple myeloma cells, and CD3 on T-cells. By binding to both, it activates T-cells to release immune signals (cytokines) and directly destroy the cancerous myeloma cells.
Positive Outcomes from Clinical TrialsThe SFDA approved Elrexfio following a comprehensive assessment of the totality of evidence, including its efficacy, safety, and quality, in line with applicable regulatory standards. Clinical data from the MagnetisMM-3 Phase 2 trial demonstrated a 58% overall response rate in patients with relapsed or refractory multiple myeloma who had received at least four prior lines of therapy. In addition, 82% of responders maintained their response for at least nine months. Elrexfio is given as a subcutaneous injection, starting with three step-up doses in the first week, followed by once-weekly dosing up to Week 24, and then once every two weeks thereafter.
Most Common Adverse Events
The most frequently reported adverse events in clinical studies included cytokine release syndrome (CRS), injection site reactions, respiratory tract infections, musculoskeletal pain, fatigue, and diarrhea.
The SFDA Orphan Drug Program Accelerates Access to Critical Therapies
This approval highlights the SFDA's commitment to enhancing access to treatment for rare and hard-to-treat diseases through the Orphan Drug Program, which aims to accelerate access to promising therapies and address unmet medical needs. An orphan drug is defined as a medication intended to treat a rare disease or condition that affects fewer than five individuals per 10,000 people in the Kingdom of Saudi Arabia.
For further information about the Orphan Drugs Guideline, please visit: (https://sfda.gov.sa/en/regulations/89310)
or contact the SFDA via email at (Designation.Drug@sfda.gov.sa)