SFDA Approves the Registration of “Qfitlia” for Hemophilia A or B
2025-12-18
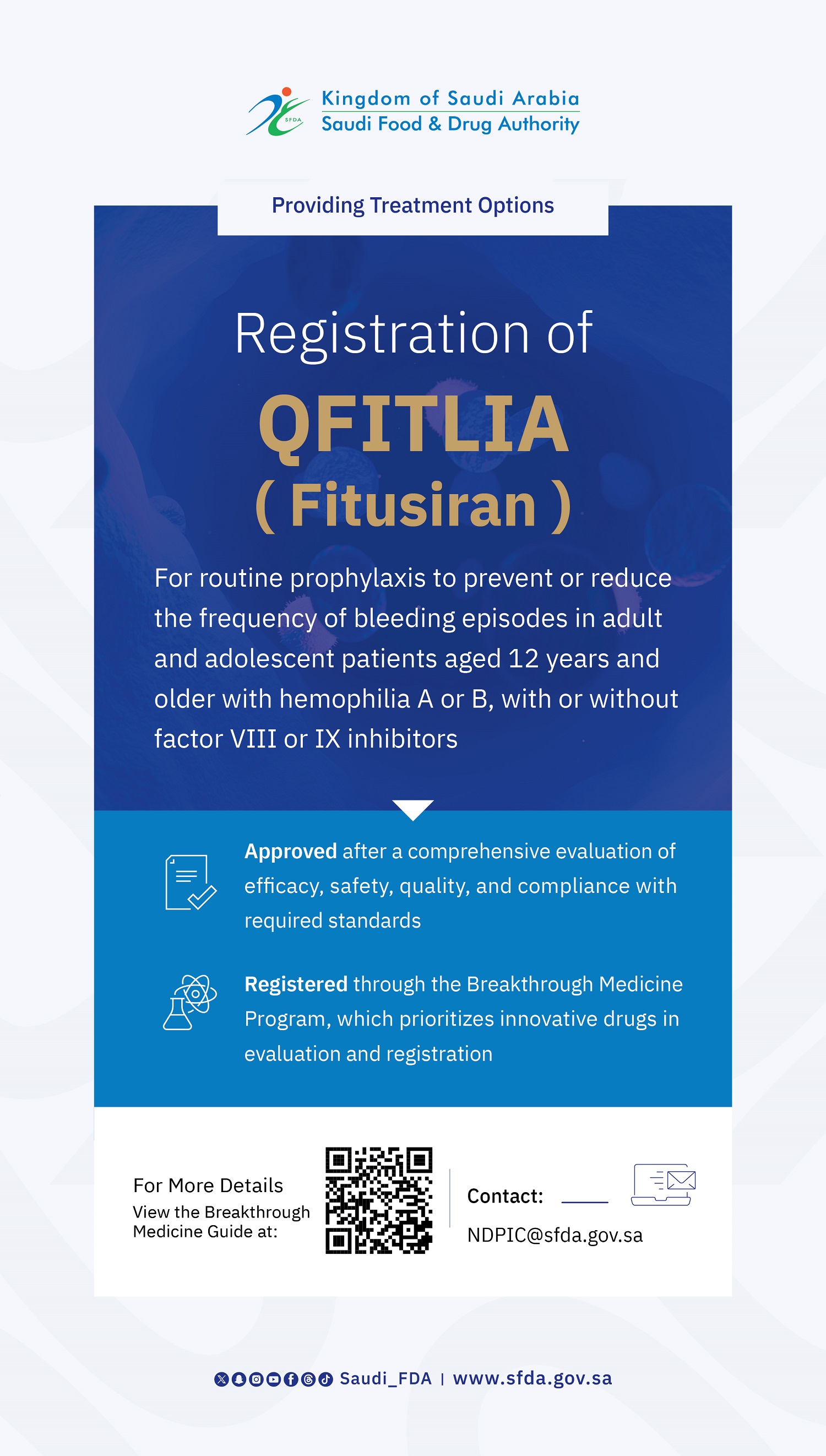
The Saudi Food and Drug Authority (SFDA) has approved the registration of Qfitlia (Fitusiran) for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adult and adolescent patients aged 12 years and older with hemophilia A or B with or without factor VIII or IX inhibitors.
This approval reflects SFDA's commitment to accelerating access to treatment options in the Kingdom of Saudi Arabia. Qfitlia was previously granted designation under the "Breakthrough Medicine Program", which aims to expedite the evaluation and availability of innovative treatments for patients with unmet medical needs.
Innovative Mechanism to Reduce Recurrent Bleeding
Qfitlia is an innovative therapy based on Small Interfering Ribonucleic Acid (siRNA). It works by targeting and degrading antithrombin (AT) messenger RNA (mRNA), leading to a reduction in plasma antithrombin levels. This mechanism enhances thrombin generation and rebalances hemostasis, thereby decreasing the frequency of bleeding in individuals with hemophilia A or B. This approach offers a promising option for managing the disease and mitigating its complications.
Positive Clinical Results Confirm efficacy and safety
The approval follows a comprehensive evaluation of the totality of evidence regarding its efficacy, safety, and quality based on established regulatory standards. Clinical trial results demonstrated the product’s significant ability to reduce bleeding episodes compared to on-demand treatments. Data showed a reduction in bleeding rates annually by nearly 90% during the follow-up period, indicating the product’s efficacy in preventing bleeding and improving overall disease control.
Safety Information and Common Side Effects
The most commonly reported side effects in clinical trials were injection-site reactions and elevated liver function tests. Consequently, patients are advised to undergo monthly liver function tests upon initiating treatment for at least six months, or following any dose adjustment. Studies also indicate potential risks associated with the medication, including thrombotic events and gallbladder disease, which require medical monitoring.
Sustained Efforts to Achieve National Objectives
This approval highlights SFDA's dedication to fostering innovation in the healthcare sector and expanding therapeutic options for patients. This aligns directly with the objectives of the Health Sector Transformation Program, a key initiative of Saudi Vision 2030, aimed at improving the quality of life and ensuring the sustainability of the national healthcare system.